HOME
PAST DIGITAL ISSUES
HIV/AIDS HOTLINES
HIV 101
POSITIVE PROFILES
ASO SPOTLIGHT
RECENT ARTICLES
HOW TO PAY FOR HIV TREATMENT AND MEDICATIONS
ADAP CRITERIA AND FORMULARIES
ASO LISTINGS
REVIEW OF HIV MEDICATIONS
ABOUT HIV POSITIVE! MAGAZINE
LINKS
SUBSCRIBE
CONTACT US
ADVERTISER INFORMATION
Biktarvy
(bictegravir/emtricitabine/tenofovir alafenamide)

DHHS Status: Recommended Initial Regimen for Most People with HIV
Manufacturer: Gilead Sciences (www.biktarvy.com) Biktarvy (www.biktarvy.com)
FDA approval date: Febuary 7, 2018
Financial Assistance: https://www.gileadadvancingaccess.com
Each Biktarvy tablet contains 50mg of bictegravir, 200mg of emtricitabine, and 25mg of tenofovir alafenamide. A standard dose is one tablet, once a day with or without food.
Review: Biktarvy is an extremely new STR, just approved in February of last year. Biktarvy combines the unboosted integrase strand transfer inhibitor (INSTI) bictegravir, with the demonstrated safety and efficacy profile of the Descovy dual nucleoside reverse transcriptase inhibitor (NRTI) backbone, and is the smallest INSTI-based triple-therapy STR available.
Biktarvy doesn’t require testing for HLA-B*5701(as is necessary with Viiv’s Triumeq), has no food intake requirements, and has no baseline viral load or CD4 count restrictions.
According to Biktarvy’s Prescribing Information, prior to or when initiating treatment with Biktarvy, healthcare providers should test for hepatitis B virus (HBV) infection and renal function, and monitor renal function as clinically appropriate during therapy.
In clinical trials through 48 weeks, no patients taking Biktarvy developed treatment resistance that was observed both in people new to therapy and those who were virologically suppressed and chose to switch regimens. Also the clinical data showed that Biktarvy’s antiviral efficacy, tolerability profile and limited drug interactions offer an effective new treatment option for people living with HIV.
More clinical trials of Biktarvy are ongoing, including a dedicated study in women and a study in adolescents and children living with HIV.
Triumeq
(dolutegravir/abacavir/lamivudine)
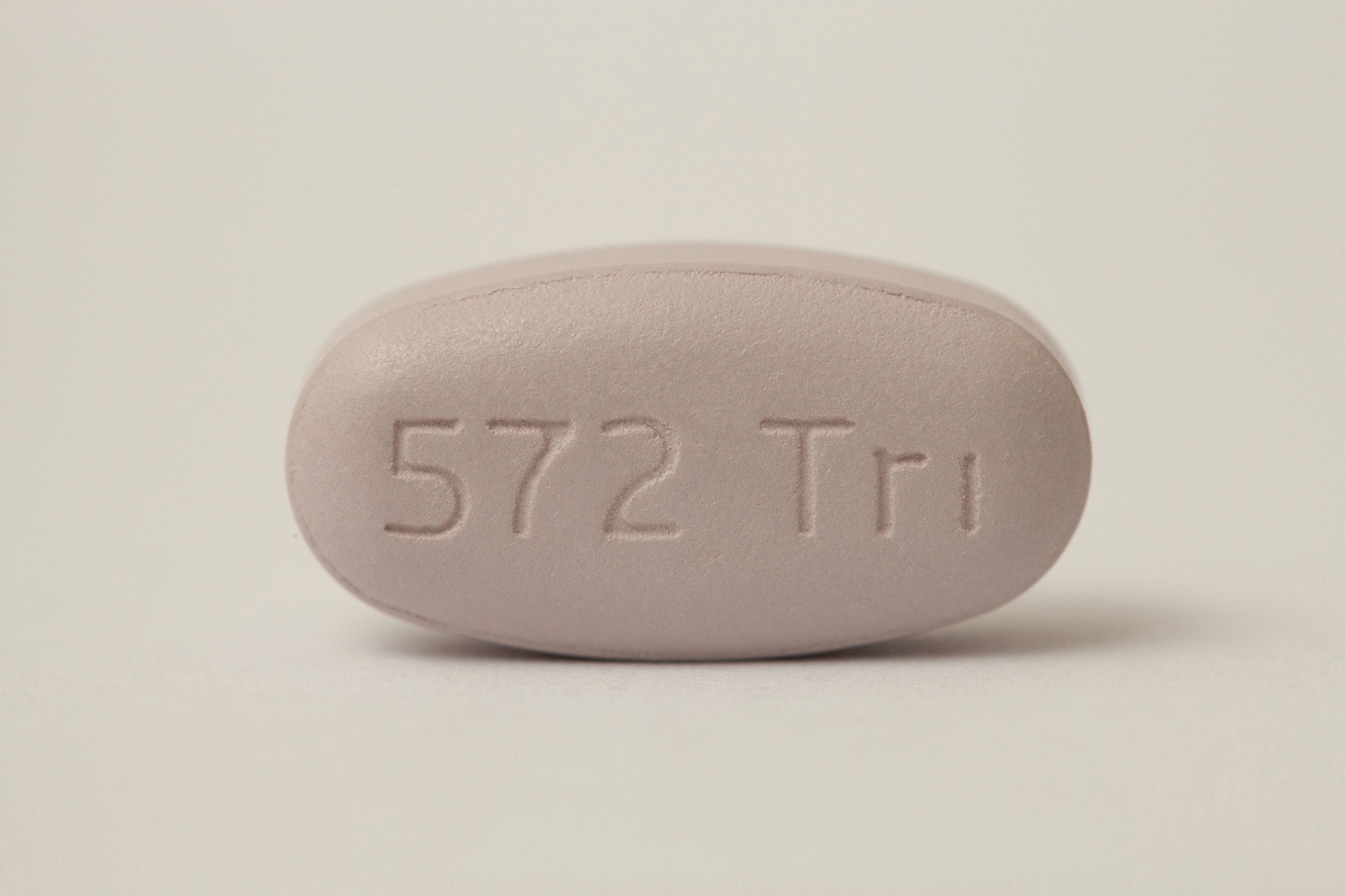
DHHS Status: Recommended Initial Regimen for Most People with HIV
Manufacturer: Viiv Healthcare (www.viivhealthcare.com) Stribild (www.triumeq.com)
FDA approval date: August 22, 2014
Financial Assistance: https://www.viivconnect.com
A standard dose of Triumeq is one tablet (50mg dolutegravir/600mg abacavir /300mg lamivudine) once a day, with or without food, for people with no evidence of Integrase Inhibitor resistance. An additional 50mg dose of Tivicay (dolutegravir) 12 hours apart from Triumeq is needed for people who have resistance to Isentress (raltegravir) or Viteka (elvitegravir).
Review: Triumeq was approved in August of 2014 and was formerly known as “572-Trii.” It is a single pill, once a day regimen for treatment of HIV and is “Recommended Initial Regimen for Most People with HIV “ by the DHHS in the current guidelines. Abacavir and Lamivudine are the combination that forms Epzicom, a Nucleoside Reverse Transcriptase Inhibitor. The third addition is Tivicay (Dolutegravir) which is an Integrase Inhibitor and was approved as a stand alone in 2013. The addition of Tivicay (Dolutegravir) is exciting though because it is what is known as a second generation Integrase Inhibitor which means it can be an effective treatment for individuals who have shown resistance to Isentress and Elvitegravir. Tivicay also doesn’t require a booster. Because it contains abacavir, potential patients should be screened for HLA- B*5701 to decrease the risk of developing abacavir hypersensitivity before beginning Triumeq.
Genvoya
(elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide)

DHHS Status: Recommended Initial Regimen in Certain Clinical Situations
Manufacturer: Gilead Sciences
(www.gilead.com),
Complera (www.genvoya.com)
FDA approval date: November 15, 2015
Financial Assistance: https://www.gileadadvancingaccess.com
Each Genvoya tablet contains 150mg elvitegravir, 150mg cobicistat, 200mg emtricitabine, 10mg tenofovir alafenamide. A standard dose is one pill, once daily with food, in adults and children 12 years of age and older.
Review: Genvoya was approved by the FDA in November of 2015 so it is a newer and advanced STR. Genvoya was moved from a DHHS “Recommended Initial Regimen for Most People with HIV” drug for first line use to “Recommended Initial Regimen in Certain Clinical Situations” this year because cobicistat can increase likelihood of drug-drug interactions. It is basically a new and improved version of Stribild which is also a DHHS recommended drug for certain clinical situations. The difference between Genvoya and Stribild is Genvoya is formulated with Tenofovir Alafenamide (TAF) while Stribild is formulated using Tenofovir Disoproxil Fumarate (TDF). This new form of Tenofovir, TAF, provides lower levels of drug in the bloodstream, but higher levels within the cells where HIV-1 replicates. It was developed to help reduce some drug side effects. Genvoya appears to be associated with less kidney toxicity (It is the only single-tablet regimen that can be given to people with impaired kidney function) and decreases in bone density than previously approved tenofovir containing regimens based on laboratory measures. Patients receiving Genvoya experienced greater increases in serum lipids (total cholesterol and low-density lipoprotein) than patients receiving other treatment regimens in studies. The use of TAF in Genvoya also allows it to be used with the Gilead hepatitis C medication Harvoni, which is not recommended with Stribild.
Stribild
(elvitegravir/cobicistat/emtricitabine/tenofovir)

DHHS Status: Recommended Initial Regimen in Certain Clinical Situations
Manufacturer: Gilead Sciences (www.gilead.com)
FDA approval date: August 27, 2012
Financial Assistance: https://www.gileadadvancingaccess.com
Each Stribild tablet contains 150mg of elvitegravir, 150mg of cobicistat, 200mg of Emtriva (emtricitabine) and 300mg of Viread (tenofovir). Standard dose is one pill, once a day and should be taken with food.
Review: Stribild is a single tablet, once a day HIV regimen and was approved by the FDA in August of 2012. It's been prescribed as a regimen for initial treatment and as regimen change from Atripla, since the side effects that some experience with Atripla are not present with Stribild. Stribild was moved from a DHHS "Recommended Initial Regimen for Most People with HIV" drug for first line use to "Recommended Initial Regimen in Certain Clinical Situations" this year because cobicistat can increase likelihood of drug-drug interactions.
Stribild was known prior to FDA approval as the “Quad” because of the combination of four drugs. The two points of differentiation from both Atripla and Complera in the one pill, once a day treatment category are two of the drugs in this combination. First, a differing component is Viteka (Elvitegravir), which is an integrase inhibitor. Viteka was actually approved for use in Stribild before it was approved as a stand alone drug although it was approved itself in September of 2014. Another differing component is Tybost (Cobicistat), which is not an antiviral HIV medication but a Pharmacokinetic (PKE) Enhancer boosting agent used to boost the levels of other medications – it’s an alternative to Norvir, another boosting agent. Tybost was also approved for use in Stribild before it acquired stand alone status in September, 2014.
Stribild is still a very strong member of the single tablet regimen market and many find it more tolerable than Atripla. Newer additions to the STR market have put a dent in it being as widely prescribed however.
Complera
(rilpivirine/emtricitabine/tenofovir)

DHHS Status: Recommended Initial Regimen for Most People with HIV
Manufacturer: Gilead Sciences
(www.gilead.com),
Complera (www.complera.com)
FDA approval date: August 10, 2011
Financial Assistance: https://www.gileadadvancingaccess.com
Each Complera tablet contains 25mg of Edurant (rilpivirine) 200mg of Emtriva (emtricitabine) and 300mg of Viread (tenofovir). Standard dose is one pill, once a day and should be taken with a meal.
Review:The current DHHS Guidelines lists Complera as a “ Recommended Initial Regimen in Certain Clinical Situations” therapy for those starting HIV treatment. Complera was the second single tablet once a day HIV regimen, having been approved by the FDA in August of 2011. It was downgraded from the previously used terms “Recommended” to “Alternative” by the DHHS in 2015 mostly due to it’s food requirement and viral load ceiling.
Complera is approved for use in new patients with viral loads up to 100,000 copies/mL and CD4 count higher than 200/mL also for treatment-experienced patients with undetectable viral loads that are changing regimens. It may also be considered as an alternative to Atripla by those people who experience the continued side effects of Sustiva (Efavirenz) the Non-Nuke component in Atripla.
Complera uses Janssen Therapeutics Non-Nuke Edurant (rilpivirine) which was approved by the FDA in May of 2011, rather than Sustiva which is the Non-Nuke used in Atripla. In testing, Edurant was seen to be as effective as Sustiva and may have fewer side effects in some patients.
Complera must be taken with a full, solid meal to allow for proper absorption - meal replacement shakes or protein drinks are not adequate substitutes and only allow for partial absorption so make sure you can plan a meal during the day.
The Complera tablet is smaller in size than Atripla, which can help people who have a tough time swallowing pills and obviously, being a single tablet once-a-day regimen, it cuts the pill burden.
Because of the fixed-dosing, people with kidney disease who may need a dosing adjustment shouldn’t take Complera.
A newer formulation of Complera, exchanging Viread (Tenofovir) for TAF which may lower kidney toxicity is available in Odefsey.
Odefsey
(emtricitabine/rilpivirine/tenofovir alafenamide)

DHHS Status: Recommended Initial Regimen in Certain Clinical Situations
Manufacturer: Gilead sciences/Janssen Therapeutics
(www.gilead.com),
Odefsey (www.odefsey.com)
FDA approval date: March 1, 2016
Financial Assistance: https://www.gileadadvancingaccess.com
Each Odefsey tablet contains 25mg rilpivirine, 200mg emtricitabine and 25mg tenofovir alafenamide. A standard dose is one tablet taken once daily, with a standard, solid meal.
Review: Odefsey is another newer addition to the STR group having been approved in 2016. As Genvoya is to Stribild, Odefsey is to Complera. Odefsey is a reformulation of Complera switching Tenofovir Disoproxil Fumarate (TDF) with Tenofovir Alafenamide (TAF). Because of the formulation with TAF, it is associated with less kidney toxicity and decreases in bone density than previously approved tenofovir (TDF) containing regimens based on laboratory testing. Odefsey was Gilead’s second TAF-based regimen to receive FDA approval.
Odefsey can be used by patients 12 years of age and older who have no antiretroviral treatment history and HIV-1 RNA levels less than or equal to 100,000 copies per mL. Odefsey is also indicated as replacement for a stable antiretroviral regimen in those who are virologically-suppressed (HIV-1 RNA less than 50 copies per mL) for at least six months with no history of treatment failure and no known substitutions associated with resistance to the individual components of Odefsey. No dosage adjustment of Odefsey is required in patients with estimated creatinine clearance greater than or equal to 30mL per minute.
Atripla
(efavirenz/tenofovir/emtricitabine)
![]()
DHHS Status: Recommended Initial Regimen in Certain Clinical Situations
Manufacturer: Gilead Sciences/Bristol-Myers Squibb www.bms.com,
Atripla (www.atripla.com)
FDA approval date: July 12, 2006
Financial Assistance: https://www.gileadadvancingaccess.com
Each Atripla tablet contains 600mg of Sustiva (efavirenz), 300mg of Viread (tenofovir) and 200mg of Emtriva (emtricitabine). Standard dose is one pill, once a day, usually taken before bedtime.
Review: Each Atripla tablet contains 600mg of Sustiva (efavirenz), 300mg of Viread (tenofovir) and 200mg of Emtriva (emtricitabine). Standard dose is one pill, once a day, usually taken before bedtime.
Atripla was downgraded from the previously used DHHS terms “Recommended” to “Alternative” by the Department of Health and Human Services (DHHS) in their guidelines since 2015. In the current guidelines, it’s status is “Recommended Initial Regimen in Certain Clinical Situations.”
Atripla was the first drug that did not need to be taken with at least one other HIV medication. For years it was considered the “gold standard” and while still prescribed (mostly in non-U.S. markets), newer regimens with equal potency and fewer side effects have taken market share away.
For the treatment-experienced, Atripla may not be an option due to drug resistance issues. Resistance can occur when medications aren’t taken as prescribed or infection can be resistant to a component in Atripla.
Some people experience psychiatric events and vivid dreams as side effects while using Atripla, which is its most common problem and for most, these diminish in severity within 4-weeks.
Atripla combines the three drugs into one pill taken once a day and cuts the pill burden, which was of course tremendously popular from its beginning in 2006.
Atripla is recommended for adults and pediatric patients 12 years old and older with a body weight of at least 88-pounds. It is not recommended for women that are pregnant or breastfeeding. Also, because Atripla is a fixed-dose combination drug, it shouldn’t be taken by people who may need dosage adjustments such as those with kidney disease.
Symfi and Symfi Lo
(Symfi (top) Symfi Lo (bottom) efavirenz/lamivudine/tenofovir disoproxil fumarate)
![]()
![]()
DHHS Status: Recommended Initial Regimen In Certain Clinical Situations
Manufacturer: Mylan Pharmaceuticals
(www.mylan.com),
Symfi (www.symfi.com) and Smyfi Lo www.symfi-lo.com
FDA approval date: February 5, 2018
Financial Assistance: https://www.activatethecard.com/symfi/#, www.activatethecard.com/symfi-lo/
Take one tablet, once daily on an empty stomach, preferably at bedtime. Each Symfi tablet contains 600mg efavirenz, 300mg lamivudine and 300mg tenofovir DF. Each Symfi Lo tablet contains a lower dose of efavirenz, 400mg, 300mg lamivudine and 300mg tenofovir DF.
Review: Take one tablet, once daily on an empty stomach, preferably at bedtime. Each Symfi tablet contains 600mg efavirenz, 300mg lamivudine and 300mg tenofovir DF. Each Symfi Lo tablet contains a lower dose of efavirenz, 400mg, 300mg lamivudine and 300mg tenofovir DF.
Symfi and Symfi Lo were approved by the FDA in February of last year. Symfi and Symfi Lo are basically alternative versions of Atripla and are the first really generic STRs. While almost the same as the branded STR, Atripla, the substitution of lamivudine (Epivir) for emtricitabine (Emtriva) makes them slightly different. The difference between Symfi and Symfi Lo is the lower dose efavirenz. See Atripla for association with nervous system side effects. These drugs, because of the generic aspect, are a less expensive regimen to enter the STR market.
Symtuza
(efavirenz/tenofovir/emtricitabine)
![]()
DHHS Status: Recommended Initial Regimen in Certain Clinical Situations
Manufacturer: Janssen Therapeutics www.janssen.com,
Symtuza (www.symtuza.com)
FDA approval date: July 17, 2018
Financial Assistance: https://www.symtuza.com/cost-savings-support
Take one tablet, once daily with food for treatment-naïve people or people with suppressed viral load on a stable HIV regimen for at least 6 months who have no known resistance to the darunavir or tenofovir components. Each tablet contains 800mg darunavir, 150mg cobicistat, 200mg emtricitabine, and
10mg tenofovir alafenamide.
Review: Symtuza was approved last year and is the first STR containing a protease inhibitor, darunavir. One of the major contributors to the development of HIV drug resistance is non-adherence. Skipping or missing doses of HIV medicine can allow the virus to multiply, which increases the risk that it could mutate and become resistant to a person’s HIV medication – or even an entire class of medications.
The U.S. Department of Health and Human Services (DHHS) guidelines recommend darunavir-based therapies for treatment-naïve patients in certain clinical situations, including when a person may have uncertain adherence, or when antiretroviral treatment (ART) should be initiated before resistance tests are available. Symtuza is the first and only complete darunavir-based single-tablet regimen (STR) that can bring clinically appropriate patients the protective barrier to resistance of darunavir right from the start in a formulation designed for improved tolerability with the convenience of an STR. Symtuza contains darunavir, which has a protective barrier to HIV drug resistance, which means it may keep on working even if the virus has changed.
Delstrigo
(doravirine/lamivudine/tenofovir DF)
![]()
DHHS Status: Recommended Initial Regimen in Certain Clinical Situations
Manufacturer: Merck & Co. www.merck.com,
Symtuza (www.delstrigo.com)
FDA approval date: August 30, 2018
Financial Assistance: https://www.activatethecard.com/7726/#
Take one tablet, once daily with or without food. Each tablet contains 100mg doravirine plus 300mg lamivudine and 300mg tenofovir DF. Approved only for adults.
Review: Delstrigo was approved in August of last year. Adults living with HIV, whose virus is suppressed with antiretroviral treatment are able to switch to Delstrigo. Currently, Delstrigo is also approved for treatment of HIV-1 infection in adults with no prior antiretroviral treatment experience.
Despite progress in the availability of HIV treatments, people living with HIV still need more options that best address their individual health needs. Delstrigo is new non-nucleoside reverse transcriptase inhibitor (NNRTI) therapy that address many of the limitations of current NNRTIs. It has a resistance profile unique from other NNRTIs, minimal drug-drug interactions, high efficacy regardless of baseline CD4+ T-cell count or high viral load, and can be taken with or without food. Delstrigo offers people living with HIV more choices to consider, including an option to change their treatment regimen. Delstrigo contains an older version of tenofovir, TDF. A safer version, TAF, is available and used in other STRs. TDF is still effective and tolerable, but TAF has less toxicity to the kidneys and bones.
Juluca
(dolutegravir/rilpivirine)

DHHS Status: Recommended as replacement regimen in those who are virologically suppressed and on a stable antiretroviral regimen for at least 6 months with no history of treatment failure.
Manufacturer: Viiv Healthcare
(www.viivhealthcare.com),
Juluca (www.juluca.com)
FDA approval date: November 21, 2017
Financial Assistance: https://www.us.juluca.com/patient-savings
Each Juluca tablet contains 50mg of dolutegravir and 25mg of rilpivirine. A standard dose is one tablet, once a day with a meal.
Review: Juluca is the first drug developed as maintenance therapy. Once someone has achieved an undetectable viral load using a 3-drug therapy method for 6-months, they can switch to this 2-drug “maintenance” therapy.
Usually when someone switches a regimen, it’s due to adverse results or side effects with their current medication. Switching to Juluca seems to benefit the patient mostly by decreasing their amount of HIV medications that they are exposed to (which certainly could be beneficial over the long haul.) Since Juluca is so new, it will be worth the watch to see how many people with current success on their existing regimens will be interested in switching.
Copyright 2019, Positive Health Publications, Inc.
This magazine is intended to enhance your relationship with your doctor - not replace it! Medical treatments and products should always be discussed with a licensed physician who has experience treating HIV and AIDS!